The European Medicines Agency’s committee had earlier announced that the vaccine is ‘safe and effective’ against the novel Coronavirus
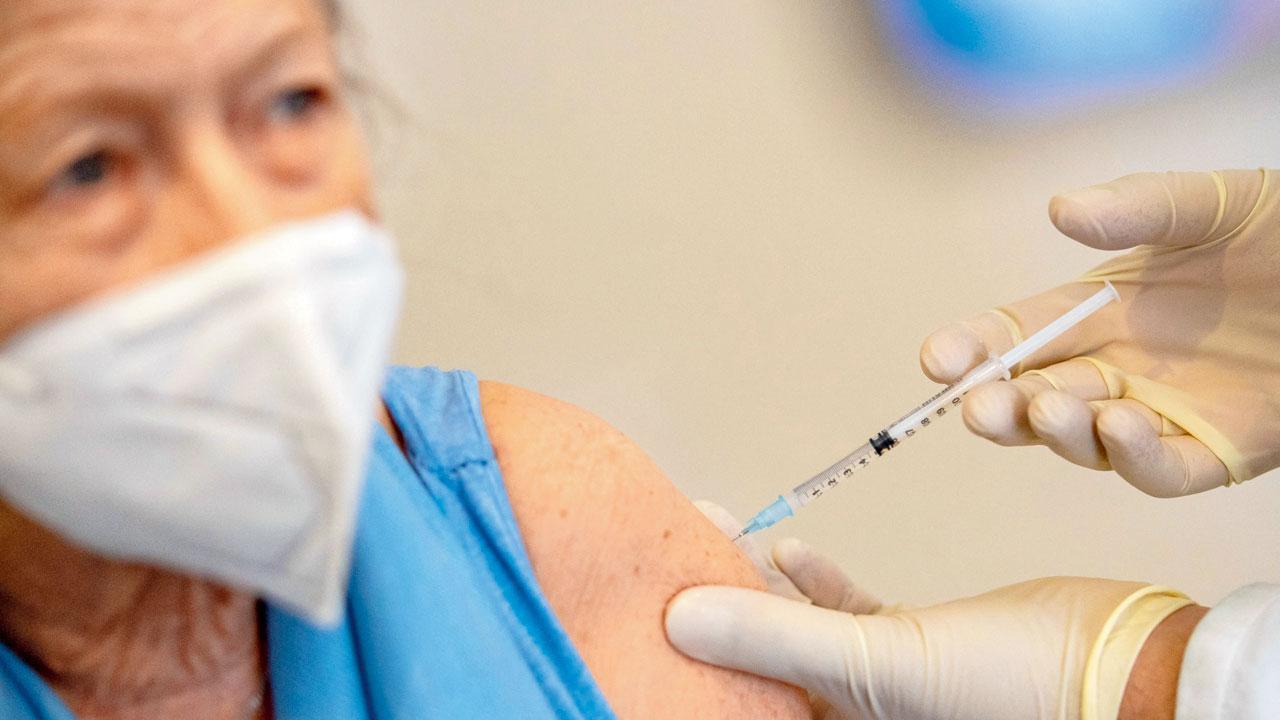
Dr Oliver Abbushi gives Dorothea Robert, AstraZeneca’s vaccine in Deisenhofen, Germany, on March 31. Germany will deploy AstraZeneca’s coronavirus vaccine for general use only for over 60 year olds. Pic/AFP
The United Kingdom has reported 30 cases of blood clots after using the AstraZeneca vaccine against coronavirus.
As per Sputnik, the Medicines and Healthcare Products Regulatory Agency, in a statement released on Thursday, said, “Up to and including 24 March, we have received 22 reports of cerebral venous sinus thrombosis (CVST) and 8 reports of other thrombosis events with low platelets, out of a total of 18.1 million doses of COVID-19 Vaccine AstraZeneca given by that date.”
In mid-March, the country had reported about only five thrombosis cases following usage of AstraZeneca vaccines, the agency further said. The European Medicines Agency’s (EMA) committee, a couple of weeks earlier, had announced that the AstraZeneca coronavirus vaccine is “safe and effective” against COVID-19. This came after some countries in the European Union temporarily suspended the use of the vaccine in mid-March as a precautionary measure based on reports of rare blood coagulation disorders in persons who had received it.
Later many leaders of EU countries including UK Prime Minister Boris Johnson got vaccinated with the AstraZeneca vaccine to restore public trust in it, upon receiving update from the agency assuring that the vaccine is not associated with an increase in the overall risk of blood clots (thromboembolic events) in those who receive it. Also, there is no evidence of a problem related to specific batches of the vaccine or to particular manufacturing sites.
Changes authorised to Moderna vaccine
The US Food and Drug Administration has authorised two changes to Moderna’s COVID-19 vaccine that can provide extra doses from each vial. The agency said late Thursday it approved new vials from Moderna that can contain up to 15 doses each, compared to original vials that held 10 doses. Additionally, regulators said providers can safely extract up to 11 doses from original 10-dose vials. The changes will be added to instructions for health care workers.
UK bans travel to Pak, Bangladesh
Pakistan and Bangladesh are among four additional countries added to the “red list” of nations from where travel to and from England is banned due to novel coronavirus. The ban will also cover the Philippines and Kenya.
China to vaccinate entire city in 5 days
A Chinese city hit by a fresh outbreak of COVID-19 began a five-day drive Friday to vaccinate its entire population of 300,000 people. State broadcaster CCTV showed people getting vaccinated in Ruili.
6,50,765
No. of new cases reported globally in the past 24 hours
12,97,37,943
Total no. of cases worldwide
28,29,863
Total no. of deaths worldwide
This story has been sourced from a third party syndicated feed, agencies. Mid-day accepts no responsibility or liability for its dependability, trustworthiness, reliability and data of the text. Mid-day management/mid-day.com reserves the sole right to alter, delete or remove (without notice) the content in its absolute discretion for any reason whatsoever
 Subscribe today by clicking the link and stay updated with the latest news!" Click here!
Subscribe today by clicking the link and stay updated with the latest news!" Click here!







